Alpha-synuclein vaccination
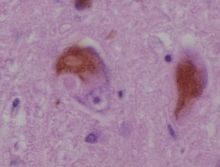
There has long been the theory that the massive accumulation of the body-specific protein "alpha-synuclein" is causally related to the death of neurons in the brain of Parkinson's patients. This is due to the fact that so-called Lewy bodies (ie small protein clumps) are formed in the brain of affected persons, which consist of more than 50% of alpha-synuclein. Since these Lewy bodies find themselves increasingly in the regions of the brain, which show precipitates in the respective Parkinsonian sufferers (for example reduced odor), it is obvious to assume that these protein collections are associated with this.
The following active ingredients are currently being developed against elevated alpha-synuclein concentrations:
The Austrian biotechnology company AFFiRiS AG based in Vienna has so far developed two vaccines (PD01A and PD03A) which are administered in the form of a few doses and, according to previous observations, lead to the formation of specific alpha-synuclein antibodies in at least one part of the subjects , In mid-2014, PDO1A successfully completed a Phase 1 clinical trial (ie testing the drug in patients with respect to tolerability and harmlessness). On 31 July 2014, AFFiRiS AG reported in a press release :
"PD01A was administered in two different doses (15 μg and 75 μg) to 12 patients in two groups. All patients received four vaccinations at monthly intervals and all conducted the study to the end. A further eight study participants received the best available medical care including a standard treatment of their symptoms and formed the control group. All subjects were regularly examined and evaluated over 12 months. In 50% of the vaccinated patients, alpha-synuclein-specific antibodies were detected in serum samples. In addition, vaccine-induced antibodies could also be found in the cerebrospinal fluid. ... The evaluation of the clinical endpoints showed a trend, consistent with all analyzed parameters, to stabilize the vaccinated subjects compared to the non-vaccinated control group ".
The second active substance of AFFiRis, named PD03A, is currently undergoing a Phase 1 klinischen Studie , of which no results have yet been published.
The Irish company Prothena Corporation plc has developed an active ingredient that reduces the amount of alpha-synuclein in the blood by up to 96% after a single dose. The drug is named PRX002 and is currently being tested in a Phase 1 clinical trial (see press release ). It is shown that PRXoo2 is well tolerated by the study participants at different doses and produces the desired reduction in alpha-synuclein levels.
Neuropore Therapies is included in a phase 1 clinical trial with the active substance NPT200-11, which binds to alpha-synuclein and thus prevents its accumulation. This approach reduces the toxic effect of alpha-synuclein and can potentially be used to both prevent and slow down Parinson's disease.
Biogen is developing a BIIB-054 antibody that binds to alpha-synuclein and enables the body to secrete it. To investigate this effect, Biogen is conducting a phase 1 clinical study that measures the content of alpha-synuclein in the nerve water of the spinal canal (spinal canal). The spinal canal is connected directly to the nerve water in the brain, which allows conclusions to be drawn about the alpha-synuclein concentration in the brain (see press message ).
Based on the results so far, it is hoped that by means of such alpha-synuclein-eliminating drugs one of the causes of the Parkinson's disease can be effectively treated and thus a slowing or a stop of the disease progress can be achieved.
The already occurring damage to the brain, for example, in the area of the substantia nigra, would have to be remedied by a cell replacement therapy in order to achieve a total cure of Parkinson's.